Searching for the unexpected in Cancer Immunology
Robust immune responses are required for the elimination of malignant cells. On the other hand, antitumor immune responses not always correlate with reduced tumor growth and increased patient survival. Moreover, immune stimulatory interventions have been shown to enhance the ability of immune effectors to eradicate some, but not all, cancer cells. Tumor cells hide from immune recognition and/or cope with immune attack. In other words, tumor cells that can successfully evade anti-tumor immunity may emerge as a consequence of adaptation to the selective pressure imposed by the immune system.
One of our major research interests focuses on “the immune regulatory mechanisms rapidly acquired by the tumor cells in response to immune reactions” and “the tumor cells that intrigue the immune cells by providing activating signals”. Our studies aim to reveal immune modulatory mechanisms that diverge the immune suppression dogma in cancer. Here, type-1 CD4 + helper T (Th1) cell subset are of special interest since anti-tumor immunity is facilitated and reinforced through the actions of these cells.
Turning Around IFN-gamma
Interferon (IFN)-gamma is the uppermost cytokine implicated in anti-tumor immunity. With its cytostatic, pro-apoptotic and immune-provoking effects, IFN-gamma plays a central role in the recognition and elimination of transformed cells. Considering well-characterized anti-tumor effects of this cytokine, many clinical trials and immunotherapy approaches have been designed to reinforce IFN-gamma-mediated immunity for different types of cancers. However, the outcomes were not satisfactory and leaded to questioning of alternative actions of IFN-gamma. Many regulatory pathways can be induced by IFN-gamma to protect the normal tissues from collateral damage and to facilitate the re-establishment of homeostasis. Nevertheless, malignant cells can take the advantage of IFN-gamma as an inducer of mediators inhibiting anti-tumor immune reactions. In addition, under the influence of tumor-derived factors, certain types of immune cells are also licensed by IFN-gamma to perform regulatory actions.
Thus, IFN-gamma occupies a fundamental role in our research projects. Whether the hypothesis is related or not to the actions of IFN-gamma, the analysis of anti-tumor reactions and the behavior of tumor cells under immune attack lead the projects towards IFN-gamma-mediated alterations on the biology of tumors.
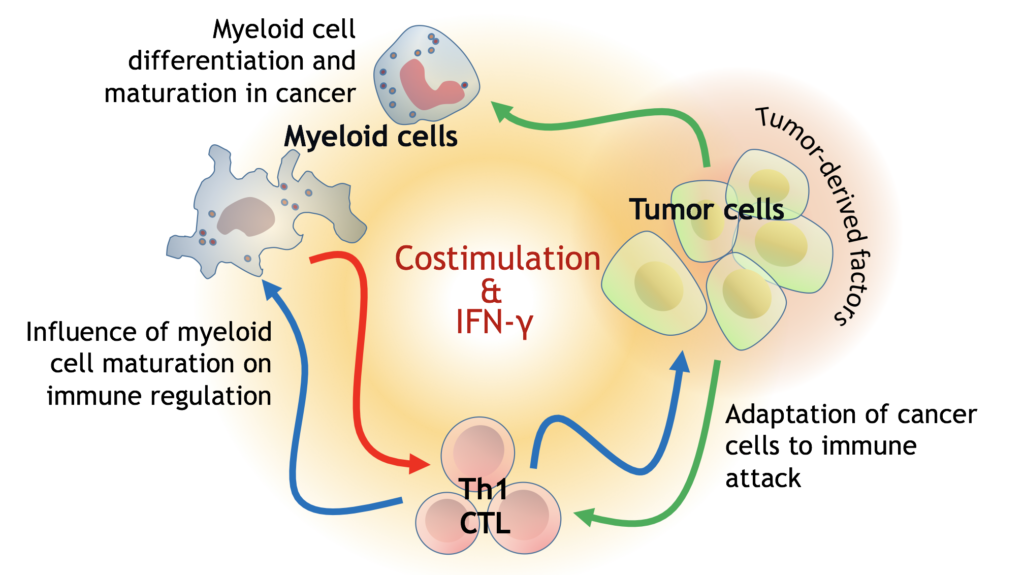
Myeloid Cells as inducers and regulators of Immunity
The cancers that possess immunostimulatory capacity regulate the features and function of myeloid cells mainly by upregulating costimulatory molecules. Analysis of the costimulatory and coinhibitory balance on myeloid cells and their influence on the activation of T-cells is one of the immunological phenomena investigated by our research group. Additionally, the relationship between costimulatory capacity of myeloid cells and T-cell hyporesponsiveness (exhaustion) is also among our research subjects; in order to comprehend how certain cancer cells survive and flourish albeit that possess capacity to strogly provoke immunity.
On the other hand, the immune system has evolved considering two basic rules: the first rule is “eradicate the foreign (non-self) if it is dangerous”, where the second rule is a caution “do not (severely) harm the host”. Considering the second rule of immune system; regulatory cells, anti inflammatory mediators and inhibitory ligands are generally found to be augmented and act as negative feedback mechanisms especially in the sites of inflammation. In the resolution phase of inflammation, the cells of myeloid origin gain immune regulatory features and play active part in termination of inflammation. Myeloid-derived suppressor cells (MDSCs) are composed of a heterogeneous group of cells that represents various stages of myeloid maturation. These cells hamper, specifically, T cell responses through inefficient immune stimulation and/or immune suppression. Our purpose is to understand the role of these myeloid cells in cancer progression and characterization of these cells.
Supporting Clinical Research on Immune-mediated Diseases
The puzzle on the paradox of resolution of inflammation and dysregulation of targeted immune response is not restricted with cancer. On the contrary, autoimmune diseases are utilized as model diseases with chronic inflammation to check what we learned in tumor immunology. Thus, patient samples from autoimmune diseases such as myasthenia gravis, multiple sclerosis, pemphigus vulgaris etc. are characterized for the immune stimulatory or regulatory molecules for the novel findings on the mechanisms of these diseases. For this purpose, our group collaborates with different clinical departments such as Department of Neurology and Department of Dermatology.
On the other hand, the immune system has evolved considering two basic rules: the first rule is “eradicate the foreign (non-self) if it is dangerous”, where the second rule is a caution “do not (severely) harm the host”. Considering the second rule of immune system; regulatory cells, anti- inflammatory mediators and inhibitory ligands are generally found to be augmented and act as negative feedback mechanisms especially in the sites of inflammation. In this resolution phase of inflammation, the cells of myeloid origin gain immune regulatory features and play active part in termination of inflammation. Myeloid-derived suppressor cells (MDSCs) are composed of a heterogeneous group of cells that represents various stages of myeloid maturation. These cells hamper, specifically, T cell responses through inefficient immune stimulation and/or immune suppression. Our purpose is to understand the role of this myeloid cells in cancer progression and characterization of these cells.
Supporting Biological Investigations of Nanomaterials and Nanotherapeutics
In addition to our primary goals in tumor immunology, our group appreciates the importance of contributing to multidisciplinary projects for the translation of science. Novel nanomaterials obtained through collaborative projects with Faculty of Pharmacy and Faculty of Science are studied for biocompatibility, toxicity, biodistribution, efficiency of cellular uptake, effects on cell proliferation and in vivo efficacy. Correspondingly, the influence of nanomaterials and nanotherapeutics on immune system functions are also founs in the scope of our research.
Striving for Proficiency in Experimental Techniques
- Flow Cytometry and cell sorting
- ELISA
- Basic and 3D Cell Culture
- Fluorescence microscopy
- In vivo Experiments with animal models
- qRT and RT PCR
- Western Blot
- Cell viability and death assays
- Molecular cloning
- In vivo imaging technology